- Home |
- Our Strength
- HIRATA's Functions to Serve Needs of Medical Industry
HIRATA's Functions to Serve Needs of Medical Industry
Sales Force

Our representatives visit medical institutions to provide and gather information on the latest medical devices. Our products and technologies are exhibited by our reps at the major medical congresses and meetings. We promote accurate and safe use of the latest medical devices, by providing easy-to-understand information on the devices.
Market Development Division
Market Development Division is in charge of investigation and analysis, product development, and market development to respond to the needs from the clinical setting.
The team is consisted of staff with different strength such as extensive sales experience or the specialty in product development.
We’re focusing together the development of medical devices that bring future and value in, while contributing the safe and secure healthcare.

Regulatory Affairs

Our Regulatory Affairs division conducts administrative procedures necessary to commercialize medical devices in Japan. We gather information needed for regulatory approval, such as the device's performance, safety and efficacy, on a global basis. We then submit them to the Japanese government in a suitable process.
Clinical Development
To obtain a regulatory approval for a new or high risk medical device in Japan, clinical data is necessary. In some cases, the regulatory approval can be obtained by utilizing clinical data acquired in other countries, but other times especially when the medical device is novel and innovative, or poses a high risk for patients, a clinical trial is required to be conducted in Japan.
In the case of clinical data not available, we will implement and managed the entire process of running a clinical trial in Japan.

Quality Assurance

We have a very robust quality assurance process in place, with substantial checks and requirements that need to be fulfilled before we distribute any device. We work with our manufacturing partners in creating standard operating procedures and optimizing processes to achieve the highest level of quality assurance, ensuring that all devices are handled with extreme care and caution.
Safety Measures
We have very strict processes and measures in place to ensure the safe and effective use of our products.
As we handle a large number of highly classified medical devices (class III and IV) that can pose a high risk, we maintain a robust system that enables prompt safety assurance measures to reduce any risk of health damage.

Medical Equipment repair and Maintenance service

Specialized staff will repair and perform regular maintenance of Medical Equipment equipment sold to customers.
We have acquired a Medical Equipment repair business based in Japan to help manage this process.
Design and Development
We continuously capture the needs from the clinical settings, carrying out in depth discussions and testing to understand how we can meet these needs through design and development to improve the devices.

Development and Production

We are engaged in the development and production of devices for endoscopic treatment (Endoscopic Injection Needle, Endoscopic Snare) and ‘Biopsy Needles’, and ‘Distal Access Catheter for Vascular’ as Medico's Hirata's own products. We also work on the development of production and sterilization processes.
-
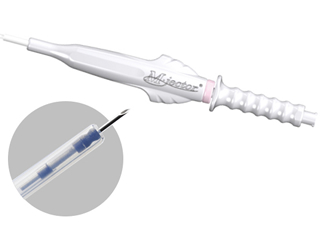
Endoscopic injection needle -
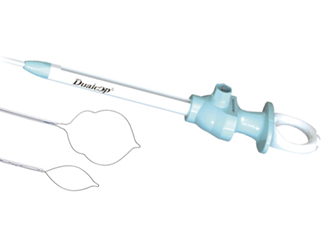
EndoscopicEndoscopic snare -

Diathermic dilator -
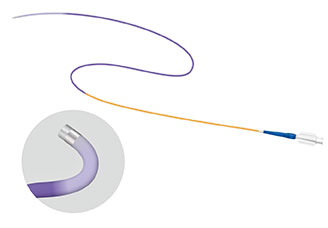
Distal Access Catheter for Vascular
Product Distribution
We have a Distribution Center and a Research facility located in Sakai city (Osaka pref.) close to Kansai International Airport. This allows for quick and efficient distribution of domestic and international products, and effectively connects us to many other markets.
For our customers to be assured, we ensure the high level stock management and reliable delivery, based on our extensive experience and thorough quality control.


MD 541367 / ISO 13485:2016
Medico's Hirata (Osaka Headquarters / Sakai facilities) has acquired the international standard "ISO 13485" certification for the quality management system for medical devices.