News
-
RED 72 launched
2023/09/01
-
sergeant support catheter launched
2023/04/01
「sergeant support catheter」
Medico’s Hirata announces the launch of “sergeant support catheter” (Trade Name:MHサージェントサポートカテーテル, Approval Number: 30500BZX00034000).sergeant support catheter has a plenty of excellent features and a wide range of sizes in order to support guidewires which are used to cross complex lesions in the lower limb peripheral vessels.
-
RED 62 launched
2023/03/01
Medico’s Hirata announces the launch of “Penumbraアスピレーション システム RED062” (Trade Name: Penumbraシステム2, Approval Number: 30500BZX00003000).
RED 62 is a system that is expected to be used in a greater variety of situations, such as "for more severely tortuous anatomy" and "with a choice of sizes for guiding systems to be used in conjunction”, compared to the existing Penumbra systems. -
HyperflexⓇ Balloon Catheter launched
2022/08/01
Medico’s Hirata announces the launch of “HyperflexⓇ Balloon Catheter ” (Trade Name : MHステントグラフトバルーンカテーテル, Approval Number : 30400BZX00069000).
Hyperflex® is a balloon catheter that can be used for post-dilation after aortic stent graft deployment. It is expandable from 10mm to a maximum of 40mm in diameter so that it can be used for thoracic and abdominal aortic stent graft grafting procedures. -
VARIXIO®Microfoam System launched
2022/07/07
Medico’s Hirata announces the launch of “VARIXIO®Pro Mag, VARIXIO®Pod Air ” (Trade Name: VARIXIOマイクロフォームシステム, Notification Number : 27B1X00058000042).
VARIXIO®Microfoam System automatically stirs sterile air and sclerosing agent to prepare foam sclerosing agent. Even with low concentrations of sclerosing agent, this system can automatically prepare a high quality microfoam sclerosing agent with fine, uniform, and long-lasting particles.
-
RED 68 launched
2022/07/01
-
Launch Information for BENCHMARK071
2022/05/01
-
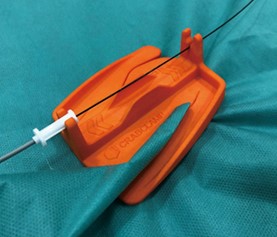
CRABCLAMP®(Launch Information)
2022/02/25
Medico's Hirata announces the launch of "CRABCLAMP®"(Manufacturer: Vascular Barcelona Devices S.I. Barcelona, Spain).
CRABCLAMP® is a product that holds catheters and guidewires in a fixed position in a simple and effective way during endovascular procedures. This effectively supports complex endovascular procedures, where many devices are used.For more information, please contact the nearest branch office of Medico's Hirata.
-
Medico's Hirata and Gellycle are expanding their alliance to commercialize Tetra gel technology as a less invasive treatment for blood circulation disorders and vascular diseases, Including Varicose Veins - A less invasive treatment will be available with just a gel injection -
2021/08/30
-
フローダイレクトマイクロカテーテル2 New Product Information
2021/07/01
Medico’s Hirata announces the launch of the “DeFrictor®Nano” (Trade name :フローダイレクトマイクロカテーテル2 , Approval number:30300BZX00064000).
DeFrictor®Nano has a redesigned lumen for improved injection control over our existing product (DeFrictor®Nano). It has also been developed with a longer flexible tip than our existing product, aiming for higher trackability in continuously tortuous blood vessels.