About Medico's Hirata
From the day of establishment in 1918, we have been at the forefront of the medical industry , bringing advanced technologies and products to our markets. In doing so, we have continued our pursuit of a social mission ― to contribute to the innovation and growth of healthcare industry.
In order to smoothly introduce innovative medical technologies from overseas, we utilize our wealth of regulatory approval know-how and our broad network of healthcare professionals that we have built up over the last century.
Our "power of connection" opens up many opportunities and gives us the potential to improve medical care.
Solid Performance and Know-how
Utilizing our trusted relationships as well as our deep network with manufacturers and healthcare professionals that we have built up the last century, we deliver advanced medical care and technologies from abroad to the Japanese market.
 |
|
|---|---|
 |
|
 |
|
 |
|
 |
|
 |
Our Record of Regulatory Applications
From 1978 to date, we have obtained more than 900 regulatory approvals and acquired expertise in resolving various issues and challenges that arise from the regulatory approval process.
Medical Devices Approval (SHONIN), Certification (NINSHO), Notification (TODOKEDE)
- In total,
- more than 900 items (from 1978 to November 2018)
more than 400 items (from 2005 to November 2018), refer to the table below for details
 |
New Approval Application | Partial Change Application(PCA) | Minor Change Notification(MCN) |
|---|---|---|---|
| 9 | 28 | 64 | |
 |
8 | 13 | 42 |
 |
26 | 31 | 149 |
 |
41 | 29 | N/A |
Our Approach to Minimally Invasive Medical Care
We make safety and reliability the first priority in all of our work and deliver medical devices to healthcare facilities to improve minimally invasive medical care with less burden on patients.
We are focused on the importance of ‘quality of life’ for our patients and strive to deliver ‘people friendly medical care’.
Contributing to the Development of Medical Care
The medical field is subject to constant change. We therefore always keep an eye out for the newest information and latest technologies.
We provide healthcare professionals with the latest information through seminars on advanced medical technologies and products, and actively participate in various conferences and symposiums.
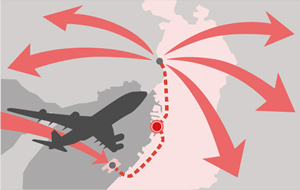
Distribution Center Connecting the World to Japan
We have a Distribution Center and a Research facility located in Sakai city (Osaka pref.) accessible to major airports in Osaka( Kansai International Airport,Osaka International Airport) that enables us to effectively import and distribute products through our domestic market.
Over the years we have developed an efficient and reliable stock management system.