Regulatory Application
In order to distribute medical devices in Japan, it is necessary to file an application for regulatory approval to the Minister of Health, Labour and Welfare, the Pharmaceuticals and Medical Devices Agency (PMDA) or the Registered Certification Bodies to obtain an approval (SHONIN) or certification (NINSHO).
We ensure to file an application appropriately utilizing our long-term trusted relationships, that we have established with relevant authorities and healthcare professionals, as well as our accumulated expertise.
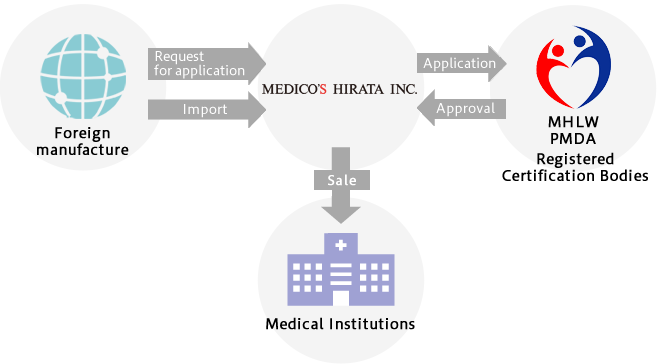
Overall flow from Manufacturing to Distribution of Medical Devices

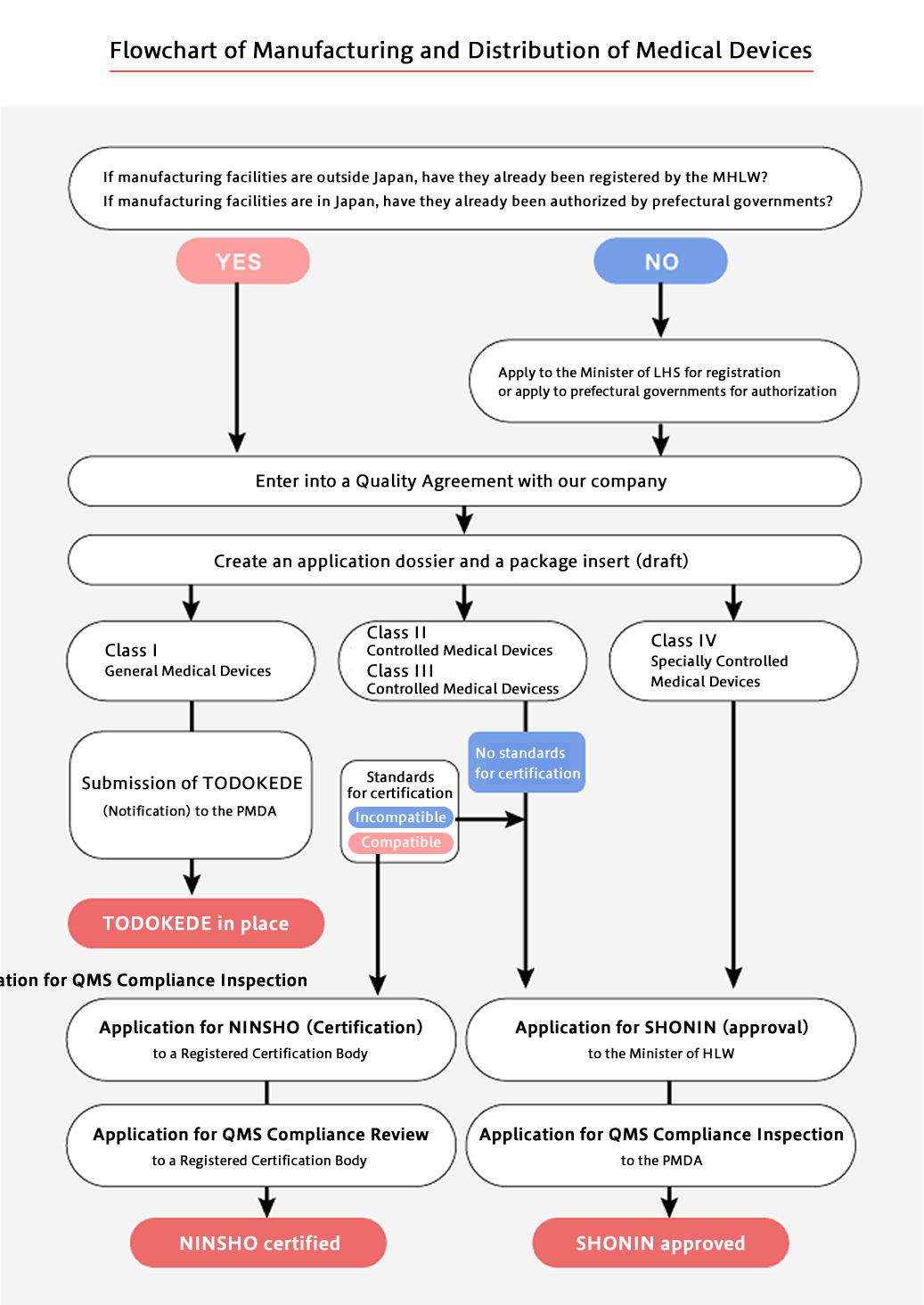
Classification of Medical Devices
| Classification | Type of Pre-market Permission | |
|---|---|---|
| Class I (General Medical Devices) |
Devices with extremely low risk to the human body in case of problems | TODOKEDE (Notification) |
| Class II (Controlled Medical Devices) |
Devices with relatively low risk to the human body in case of problems | NINSHO (Certification) or SHONIN (Approval) |
| Class III (Specially Controlled Medical Devices) |
Devices with relatively high risk to the human body in case of problems | SHONIN (Approval) or NINSHO (Certification) |
| Class IV (Specially Controlled Medical Devices) |
Devices highly invasive to patients and with life-threatening risk in case of problems | SHONIN (Approval) |
Appropriate Regulatory Applications

We gather information needed for regulatory approval, such as the device's performance, safety and efficacy, on a global basis. We then document the information and submit them to the Japanese public administration in a suitable process.
In order to file an application at an appropriate timing in an appropriate manner’, we effectively manage the whole timeline from product development to launch, while working in tandem with other relevant divisions and the manufactures to proceed the application process.
From Clinical Trial Design Planning to Protocol Creation

To obtain a regulatory approval for a new or high risk medical device in Japan, clinical data is necessary.
In some cases, the regulatory approval can be obtained by utilizing clinical data acquired in other countries, but sometimes especially with novel or high risk medical devices, a clinical trial is required to be conducted in Japan.
In the case where clinical data is not available, we will implment and managed the entire process of running a clinical trial in Japan.
Our Approach to Global Clinical Trials
We are capable of implementing a global trial as an In-country Clinical Caretaker (ICCC) in cooperation with overseas sponsors, so that the cutting-edge devices from overseas can be promptly introduced into the Japan market. In addition, we offer proposals to overseas sponsors to represent actual conditions of medical treatment in Japan and in accordance with Japanese regulations in order to develop a protocol that can accommodate a global clinical trial.
Our company has extensive experience in clinical trials for devices such as Coronary stent, Vascular Graft, Endovascular Graft, Endovascular Laser, Intracranial Stent, SFA stent, and Transcatheter Aortic Valve.
Our Record of More Than 900 Approvals

We have obtained more than 900 regulatory approvals from 1978 to date, gaining substantial expertise in resolving various issues and challenges that arise from the approval process.
Our Record of Regulatory Approvals
HIRATA's Functions to Serve Needs of Medical Industry
See more of HIRATA's functions to serve needs of medical industry.