Designing and Manufacturing Medical Devices
We design and develop products based upon the new product needs from medical sites.
When converting the ideas into a product, our aim is to promptly make it available at the clinical setting either as our own or as OEM product, while we do not compromise to pursue the functions and features with great usability.
Design and Development to Address the Needs
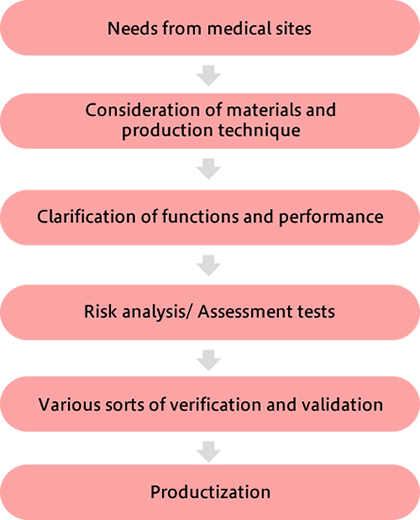
Our Design and Development division is a dedicated team to grasp customers' needs, determine the best material or production technique to meet the needs, and to turn the needs into a product upon clarification of feasible functions and performance. Once the product is under development, different experts in each field would then continue conducting risk analysis and assessment tests from various aspects in a repetitive manner before the verification and validation phase, to finally materialize the outcome.
Aiming High to Offer High Quality and Reliable Products
The needs from medical sites keep evolving and the hopes and expectations consistently get higher. The key factors to respond these, are the pursuit of functions and clinical performance, together with the latest materials and production technique. To obtain such materials and technique, we diligently collect information from across the globe, and we take on challenges to realize products in the combination of the latest and the best way possible.
For vascular products, we design and develop through OEM products.
-
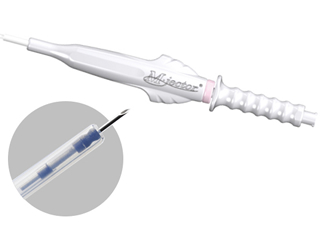
Endoscopic injection needle -
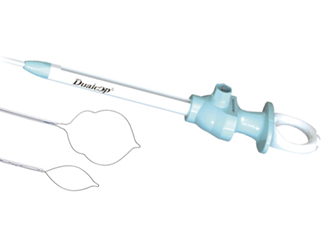
Endoscopic snare -

Diathermic dilator
Made In Japan
We have uncompromising stance when it comes to quality. As we carry out rigorous inspection on every single unit of our own products to be used for patients, we are achieving the quality with almost zero defects. We feel sense of pride in supplying our made-in-Japan products that are high quality, safe and reliable, to medical sites.
Enhancing Proper Quality System

We are engaged in the quality check, inspection of incoming goods, and the judgement whether the products are suitable for release, ensuring that the entire process from the incoming to shipment of the products is appropriate and confirm our evaluation in accordance with SOPs, documentation, and on-site inspections. Not only we work on validation and evaluation of the quality system of manufacturers, but we work together with them in creating standard operating procedures or by making proposals to further optimize the operation, with the common aim to maintain the quality of the product in the best way possible.
Market Oriented-Test Process

We own the Hirata Lab within the premises where we replicate the environment of an angio suite of clinical setting. It is equipped with a DSA, an operation table, an automatic contrast media injector as well as the sophisticated blood vessel models and we are conducting the necessary.
This way, the Lab is serving effectively and critically to enable us to launch the clinically desired medical devices into the market at early stage.
The Hirata Lab is also utilized as a device training venue for the healthcare professionals or for our own employees.
Safe and Reliable Products to Medical Sites
In the light of 1) product quality assurance and 2) safe use, we constantly monitor our products released to the market.
Having conducted a post marketing surveillance (PMS) of 3000 cases is one example. But our mission is to prevent the occurrence of health damage. And our way is to work with healthcare professional to provide the patients with our service called "safety".
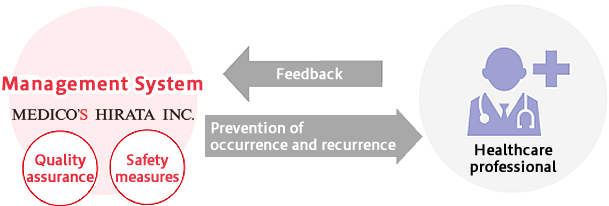
With all the processes being implemented in accordance with HMS (Hirata Management System*), we endeavor for the prevention of occurrence and recurrence of clinical events and complain.
* Medico's Hirata's one-of-a-kind management system complying with ISO13485, QMS, and GVP etc.
HIRATA's Functions to Serve Needs of Medical Industry
See more of HIRATA's functions to serve needs of medical industry.