News
-
Medico’s Hirata and Gellycle, a bio-venture company from the University of Tokyo, launch a joint development project for the practical use of Tetra-gel in medical treatments
2021/04/03
-
oceanus 14 RX PTA balloon dilatation catheter Launch Information
2021/01/05
Medico’s Hirata announces the launch of “oceanus 14 RX PTA balloon dilatation catheter” (Trade Name: MHオセアナスPTAバルーンカテーテル1, Approval Number: 30200BZX00324000).
Oceanus 14 RX PTA balloon dilatation catheter has a hydrophilic coating (HYDRAX PLUS), iVascular S.L.U. ’s proprietary technology (manufacturer LIFE VASCULAR DEVICES BIOTECH S.L.), which is expected to improve device crossability in complicated lesions.
iVascular S.L.U. is a global company headquartered in Spain that supplies endovascular devices to approximately 70 countries worldwide. We have an exclusive distribution agreement with iVascular S.L.U. in Japan. In Japan we provide their products as a distributor. We are confident that the we can further contribute to endovascular treatments in the future.
-
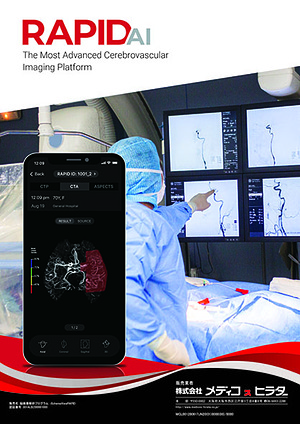
iSchemaViewRAPID Information
2020/09/29
Medico’s Hirata announces the distribution of 「 iSchemaViewRAPID 」(Trade Name:脳画像解析プログラム iSchemaViewRAPID、 Certification Number : 301ALBZI00001000)
iSchemaViewRAPID employs the technology based on clinical evidence. It is automated imaging analysis software, delivering maps to help physicians in minutes from receipt of a scan such as CTP, MRI and so on. Its technology has been used for patient selection in over 14 multi-site clinical trials including DAWN, DEFUSE3, EXTEND-IA, and currently it is being installed in over 1,500 sites in 50 countries in the world.
In Japan, Micron, Inc. received the medical device certificate (NINSHO) in June 2019. Our distribution started on March 6, 2020. -
Fine025 flex type(Disposable Electricity Dilator)(self-developed product) launched
2019/08/01
-
Ruby GEN2 EMBOLIZATION SYSTEM launched
2019/03/01
Medico’s Hirata announces the launch of Penumbra Inc. “Ruby GEN2 EMBOLIZATION SYSTEM”(Trade Name: Penumbra PC400 コイルシステム、Approval Number: 22400BZX00294000)
Ruby GEN2 coil is a large–volume coil with a primary diameter of 0.020 inch. A size range of up to 60cm in length and 40mm in diameter makes more choices available in various coil embolization . In addition, a flexible coil design achieves higher packing densities.
-
Fine025 (Disposable Electricity Dilator)(self-developed product) launched
2018/11/01
弊Medico’s Hirata announces the launch of “Fine025” (self-developed product; Trade name:ディスポーザブル内視鏡通電ダイレータ, Certification Number: 230ALBZX00020000).
The thinned distal end (1.0 mm) and appropriate stiffness of the sheath are expected to make it possible to smoothly pass across a stricture.
The tapered electrode aims at a reliable procedure while decreasing possible injury to the surrounding tissues.
-
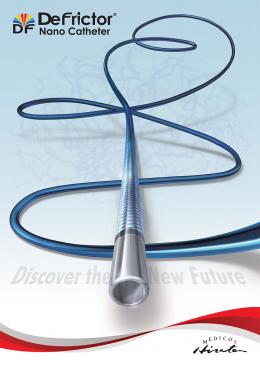
Flow Directed Microcatheter launched
2018/04/02
Medico’s Hirata announces the launch of “DeFrictor®” (Trade Name: フローダイレクトマイクロカテーテル, Approval Number 22900BZX00258000).
DeFrictor Nano Catheter is designed with 1.3 Fr low profile distal shaft for use in small vessels. The braide (in the whole shaft) protects inner lumen integrity and provides high or excellent pushability across the peripheral vessels. These features permit approach to small vessels which were difficult to be accessed before and sharply angulated side branches.
-
“RINGLIGHT SLIM FIBER PROBE” launched
2018/02/05
Medico’s Hirata announces the addition of the “RINGLIGHT™ SLIM FIBER PROBE”, a thin laser fiber, to the lineup of LSO Medical’s “Endothermelaser™ 1470” (Trade Name:LSO1470レーザー, Approval Number:22700BZX00311000).
Endovenous laser ablation for which the Endothermelaser™ 1470 is intended to be used to treat varicose veins is a treatment to block the vein by omnidirectionally ablating the venous wall to shrink with a laser beam from the laser fiber inserted into the main trunk of the saphenous vein. It is a relatively new therapy approved to be reimbursed in 2011 in Japan, drawing attention these days as a minimally invasive procedure which allows varicose veins to be treated with a small incision.
This latest addition, the “RINGLIGHT™ SLIM FIBER PROBE”, is made thinner by approx. 2F than our conventional laser fiber, which is expected to make treatment even less invasive.
-
Penumbra Inc. “Reperfusion Catheters (ACE68)” launched
2017/11/25
-
Flex type and Fitto type added to EGoist Series
2017/08/29
Medico’s Hirata announces that Flex type and Fitto type were added to EGoist®, a dedicated guide wire for stent grafts (Trade Name: MH心臓・中心循環系用ガイドワイヤ, Approval Number: 22500BZX00510000).
EGoist® Flex & Fitto Interventional Guide Wire features both softness and stiffness with Super Long Tapered Core which added trackability and flexibility to conventional supportability.