News
-
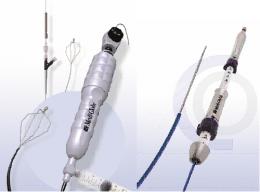
PTA balloons for peripheral vascular intervention launched
2014/07/07
-
Announcement of transfer of distribution and sales rights of Cook products (Peripheral Intervention and Interventional Radiology) to be transferred to Cook Japan
2014/03/04
Following the previous transfer of Cook aortic intervention and endoscopy products on April 1, 2012, Medico’s Hirata is now to transfer the distribution and sales rights of Cook peripheral intervention and interventional radiology products, as of April 1, 2014.
Cook peripheral intervention and interventional radiology products will continue to be available through Medico’s Hirata until March 31, 2014. After April 1, however, all the Cook products sold by us so far will be handled by Cook Japan. We look forward to your kind understanding.
We would like to take this opportunity to express that your continued purchase of Cook products through us over the past years is sincerely appreciated. As this transfer will enable us to add more advanced and high-quality products to our portfolio, we will commit to positively provide more useful medical devices. Your continued and further patronage and assistance would be highly appreciated.
-
Press release announcing the nation’s first Japan-US joint clinical trial in the field of neuroendovascular therapy available
2013/09/30
Medicos Hirata announces the commencement of its global clinical trial of a new stent for treating wide-neck intracranial aneurysms jointly conducted with Penumbra, Inc. For further details, please refer to the press release below.
http://www.businesswire.com/news/home/20130930006724/ja/
-
Exclusive distribution agreement with Lombard Medical Technologies PLC
2013/02/20
Medico's Hirata concluded an exclusive distribution agreement for Japanese market with Lombard Medical Technologies PLC( which headquarter is in Britain), for their flagship product; endovascular stent graft to treat abdominal aortic aneurysm(AAA).
For more details, please refer their homepage.http://www.lombardmedical.com/
-
BONASTENT released
2013/01/18
-
Exclusive distributor agreement with Medi-Globe GmbH
2012/08/14
Medico’s Hirata entered into an exclusive distributor agreement with Medi-Globe GmbH for the Japanese market.
Medi-Globe GmbH, headquartered in Germany, sells endoscopy-related devices in approx. 50 countries around the world.
Medico’s Hirata will serve as a sole distributor of Medi-Globe GmbH products in Japan.
We believe that we will be able to help better endoscopic testing and treatment in the gastrointestinal, bilio pancreatic and respiratory fields in the future. -
New convex-type ultrasound endoscope (HOYA Corporation) launched
2012/08/01
Medico’s Hirata started distributing the new endoscopic ultrasound scope EG-3270UK (manufactured by PENTAX Life care, a division of Hoya Group) on August 1, 2012.
We hope that this convex-type device with a slim insertion tube compared with the conventional convex-type ultrasound endoscopes, will achieve not only mitigation of patients’ pain but also smooth testing by healthcare professionals. -
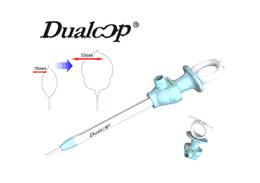
Launch of Dualoop Snare
2011/10/01
Medico’s Hirata announces the launch of its self-developed “Dualoop ® Snare”, ディスポーザブル内視鏡高周波スネア(Certification Number 223ADBZX00031000) .This product makes possible 2 loop sizes (16mm and 33mm) in a single device to treat various sized lesions in EMR procedures. The wire has an optimal stiffness to capture polyps easily, and to maintain flatness while grasping.
-
Reimbursement of Penumbra Systems
2011/10/01
Penumbra System (Approval Number 22300BZX00269000; Penumbra Inc., USA), a clot retrieval device for acute stroke will be reimbursed.
The Penumbra System received marketing approval by the Japanese Ministry of Health, Labor and Welfare on June 9th, 2011. It treats acute stroke by inserting a catheter connected to the aspiration pump under radioscopy, through a patient’s femoral artery, and aspirating/retrieving clots in the affected area.
This product is indicated for acute stroke patients presented within 8 hours of symptom onset, who are contraindicated to t-PA or did not achieve revascularization by t-PA administration. -
Concluded an exclusive distributorship agreement with STARmed Co.,Ltd.
2011/02/07
We concluded an exclusive distributorship agreement in Japan with STARmed Co., Ltd. STARmed is a manufacturer/seller of radiofrequency ablation devices.
For details, please refer to STARmed’s company website.